What’s that got to do with the silicon here?
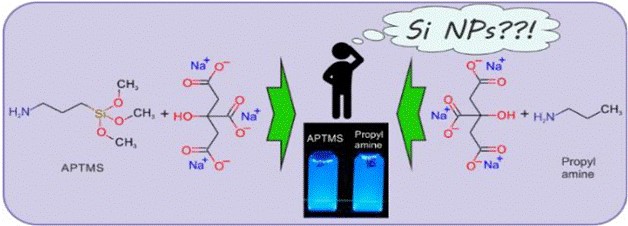
Due to their unique optical characteristics, silicon-based colloidal nanoparticles (NPs) are well known to be extremely promising for various multidisciplinary applications, such as, bio-imaging, drug delivery, photo-induced therapy and many others. In 2013, a new aqueous synthetic approach based on one‐pot synthesis of fluorescent Si NPs using of (3‐aminopropyl)trimethoxysilane (APTMS) as the silicon source and trisodium citrate dihydrate (Na3Cit) was reported by Zhong et al. This work has attracted a great deal of attention from the scientific community since 2013 and has accumulated 227 citations according to Google Scholar (June 6, 2019), 203 citations according to Scopus (May 18, 2019), or 191 citations according to SciFinder (June 6, 2019). However, taking into account common accepted knowledge in the field of silicon chemistry, low temperature formation of crystalline Si NPs al. from the water solutions of alkoxysilanes seems very doubtful.
In order to check the assumption expressed by Zhong et al. regarding the formation of Si NPs, the control reactions between sodium citrate and organic amine (one containing silicon, APTMS, and one without silicon, n-propylamine) have been carried out. The steady-state and time-resolved PL, PL excitation and optical absorbance spectra were found to be identical, independent of the presence or absence of silicon in the organic amine. All these experimental data strongly emphasize the similarity of the products obtained during the reactions between sodium citrate and organic amines, which of course includes the sample containing APTMS (the Si-containing amine), and the silicon-free sample that only has n-propylamine.
Thus, we can conclude that silicon is not responsible for the optical properties of the obtained reaction products. Considering our experiments, as well as taking into account the general chemical properties of silicon, it seems reasonable to assume that the formation of Si NPs under the hydrothermal conditions used by Zhong et al. seems rather doubtful and improbable. Thus, the most acceptable statement is that the PL properties of the reaction products reported by Zhong et al. are rather similar to those of different carbon nanoparticles and organic dyes.
One of the most important goals of our work is to prevent huge wave of misinterpretations caused by the article of Zhong et al. However, the remarkable photoluminescent properties of the synthesized chemical species may provide access to useful organic carbon-containing particles that can be successfully used for numerous interdisciplinary applications, such as, for example, those aimed by our European CARTHER project (proposal #690945) of Marie Skłodowska-Curie Research and Innovation Staff Exchange program (Horizon 2020 Framework Programme).
Référence: B. Oliinyk, D. Korytko, V. Lysenko and S. Alekseev, Are fluorescent silicon nanoparticles formed in a one-pot aqueous synthesis?, accepted for publication in Chemistry of Materials, 2019, DOI: 10.1021/acs.chemmater.9b01067
Collaboration: Sergei Alekseev’s team, Faculty of Chemistry, Kiev T. Shevchenko National University