Keys to control the nanocrystalline growth of visible bandgap multiferroic BiFeO3
BiFeO3 is the most promising ferroelectric candidate in different optical multifunctional applications because of its relatively small band gap (Eg = 2.6-2.8 eV) in comparison to other classical ferroelectric oxides. Moreover, its large polarization value could be beneficial to optical applications as not only a wider part of the sunlight spectrum could be exploited thanks to the low bandgap, but also the internal electric field could contribute to enhance the separation of photogenerated charge carriers, which can be exploited in photocatalytic and photolysis processes under visible light irradiation.
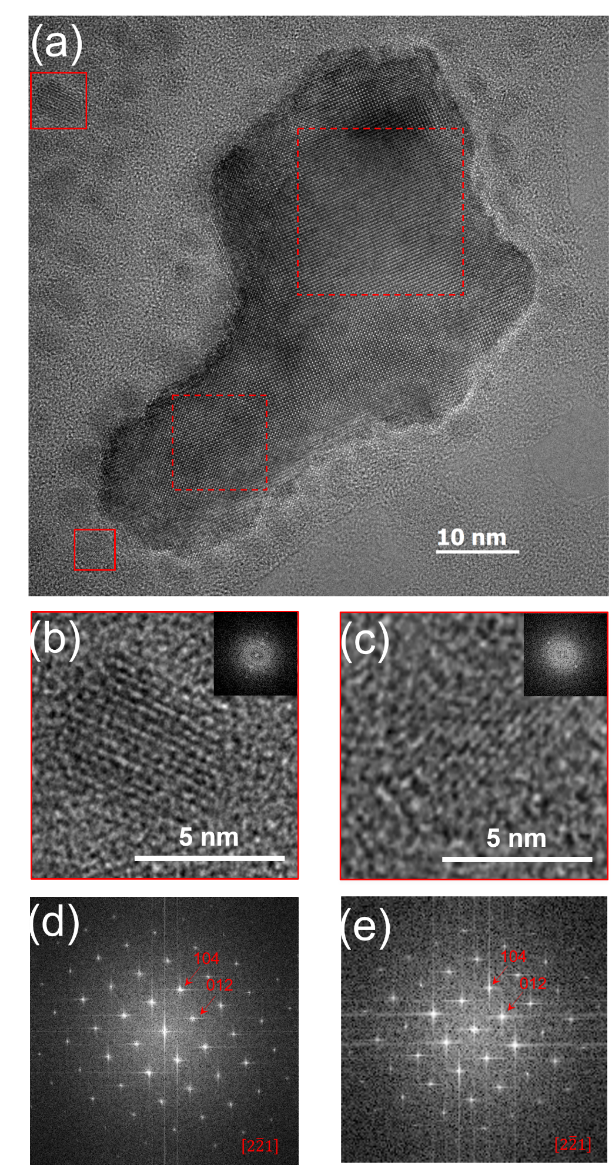
In this framework, we use wet-chemical routes and low temperature treatments to synthesize single-phase BiFeO3 nanoparticles. We have determined the different phase transformation processes happening at the nanoscopic level through calorimetry, seeking to identify the different steps in the evolution from amorphous to complete crystalline matter, and finally set a reproducible protocol to obtain multiferroic BiFeO3 nanoparticles. Two crystallization steps are responsible of the complete single BiFeO3 nanoparticle formation, which can be thermally separated. First crystallization step happens at relatively low temperature, below 450C, from nanocrystal nucleation, which is followed by single nanocrystal growth. At higher temperature, the nanocrystallite coalescence leads to polycrystalline nanoparticles assemblying (Figure 1). Finally, using high resolution transmission electron microscopy (Figure 2), we prove that crystal nucleation happens within the amorphous-rich area in multiple seeds, leading to the formation of single crystalline nanoparticles with no preferential faceting, in agreement with the deduced structural analysis using x-ray diffraction.
Contacts
Ingrid C. INFANTE –
Xiaofei BAI –
Collaborations
Matthieu BUGNET (MATEIS, UMR 5510 CNRS, INSA Lyon UCBL Lyon 1, Villeurbanne, France)
Carlos FRONTERA (Institut de Ciència de Materials de Barcelona CSIC, Bellaterra 08193, Spain)
Pascale GEMEINER (SPMS, UMR 8580 CNRS, CentraleSupélec, Gif-sur-Yvette, France)
Jérôme GUILLOT and Damien LENOBLE (Luxembourg Institute of Science and Technology, Materials Research and Technology Department, Belvaux, Luxembourg)
Acknowledgements
This work was supported by the IDEXLYON (ANR-16-IDEX-0005) FERLIGHT project from the Université de Lyon. Jêrome Guillot; Damien Lenoble and Ingrid C. Infante express thanks for the FNR funding provided by the INTER/Mobility FOXCAT Project No. 15/9887562.
Reference
Inorg. Chem. 2019, 58, 17, 11364-11371

Figure 1: Sketch of the BFO nanoparticle crystallization process following the present sol-gel method.